Enlisting Monoclonal Antibodies in the Fight Against COVID-19
Posted on by Dr. Francis Collins
We now know that the immune system of nearly everyone who recovers from COVID-19 produces antibodies against SARS-CoV-2, the novel coronavirus that causes this easily transmitted respiratory disease [1]. The presence of such antibodies has spurred hope that people exposed to SARS-CoV-2 may be protected, at least for a time, from getting COVID-19 again. But, in this post, I want to examine another potential use of antibodies: their promise for being developed as therapeutics for people who are sick with COVID-19.
In a recent paper in the journal Science, researchers used blood drawn from a COVID-19 survivor to identify a pair of previously unknown antibodies that specifically block SARS-CoV-2 from attaching to human cells [2]. Because each antibody locks onto a slightly different place on SARS-CoV-2, the vision is to use these antibodies in combination to block the virus from entering cells, thereby curbing COVID-19’s destructive spread throughout the lungs and other parts of the body.
The research team, led by Yan Wu, Capital Medical University, Beijing, first isolated the pair of antibodies in the laboratory, starting with white blood cells from the patient. They were then able to produce many identical copies of each antibody, referred to as monoclonal antibodies. Next, these monoclonal antibodies were simultaneously infused into a mouse model that had been infected with SARS-CoV-2. Just one infusion of this combination antibody therapy lowered the amount of viral genetic material in the animals’ lungs by as much as 30 percent compared to the amount in untreated animals.
Monoclonal antibodies are currently used to treat a variety of conditions, including asthma, cancer, Crohn’s disease, and rheumatoid arthritis. One advantage of this class of therapeutics is that the timelines for their development, testing, and approval are typically shorter than those for drugs made of chemical compounds, called small molecules. Because of these and other factors, many experts think antibody-based therapies may offer one of the best near-term options for developing safe, effective treatments for COVID-19.
So, what exactly led up to this latest scientific achievement? The researchers started out with a snippet of SARS-CoV-2’s receptor binding domain (RBD), a vital part of the spike protein that protrudes from the virus’s surface and serves to dock the virus onto an ACE2 receptor on a human cell. In laboratory experiments, the researchers used the RBD snippet as “bait” to attract antibody-producing B cells in a blood sample obtained from the COVID-19 survivor. Altogether, the researchers identified four unique antibodies, but two, which they called B38 and H4, displayed a synergistic action in binding to the RBD that made them stand out for purposes of therapeutic development and further testing.
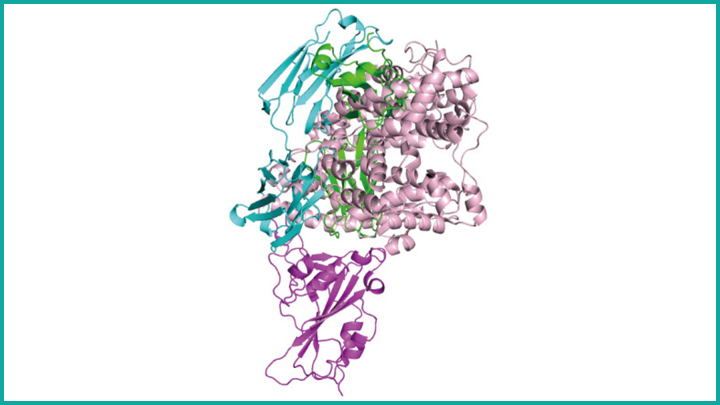
To complement their lab and animal experiments, the researchers used a particle accelerator called a synchrotron to map, at near-atomic resolution, the way in which the B38 antibody locks onto its viral target. This structural information helps to clarify the precise biochemistry of the complex interaction between SARS-CoV-2 and the antibody, providing a much-needed guide for the rational design of targeted drugs and vaccines. While more research is needed before this or other monoclonal antibody therapies can be used in humans suffering from COVID-19, the new work represents yet another example of how basic science is expanding fundamental knowledge to advance therapeutic discovery for a wide range of health concerns.
Meanwhile, there’s been other impressive recent progress towards the development of monoclonal antibody therapies for COVID-19. In work described in the journal Nature, an international research team started with a set of neutralizing antibodies previously identified in a blood sample from a person who’d recovered from a different coronavirus-caused disease, called severe acute respiratory syndrome (SARS), in 2003 [3]. Through laboratory and structural imaging studies, the researchers found that one of these antibodies, called S309, proved particularly effective at neutralizing the coronavirus that causes COVID-19, SARS-CoV-2, because of its potent ability to target the spike protein that enables the virus to enter cells. The team, which includes NIH grantees David Veesler, University of Washington, Seattle, and Davide Corti, Humabs Biomed, a subsidiary of Vir Biotechnology, has indicated that S309 is already on an accelerated development path toward clinical trials.
In the U.S. and Europe, the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) partnership, which has brought together public and private sector COVID-19 therapeutic and vaccine efforts, is intensely pursuing the development and testing of therapeutic monoclonal antibodies for COVID-19 [4]. Stay tuned for more information about these potentially significant advances in the next few months.
References:
[1] Humoral immune response and prolonged PCR positivity in a cohort of 1343 SARS-CoV 2 patients in the New York City region. Wajnberg A , Mansour M, Leven E, Bouvier NM, Patel G, Firpo A, Mendu R, Jhang J, Arinsburg S, Gitman M, Houldsworth J, Baine I, Simon V, Aberg J, Krammer F, Reich D, Cordon-Cardo C. medRxiv. Preprint Posted May 5, 2020.
[2] A noncompeting pair of human neutralizing antibodies block COVID-19 virus binding to its receptor ACE2. Wu Y. et al., Science. 13 May 2020 [Epub ahead of publication]
[3] Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Pinto D, Park YJ, Beltramello M, Veesler D, Cortil D, et al. Nature. 18 May 2020 [Epub ahead of print]
[4] Accelerating COVID-19 therapeutic interventions and vaccines (ACTIV): An unprecedented partnership for unprecedented times. Collins FS, Stoffels P. JAMA. 2020 May 18.
Links:
Coronavirus (COVID-19) (NIH)
Monoclonal Antibodies (National Cancer Institute/NIH)
Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV)
NIH Support: National Institute of Allergy and Infectious Diseases; National Institute of General Medical Sciences

why does it take so long?
where are the clinical tests in humans?
why isn`t that stuff immediately put into patients arriving at clinics because a severe course of the disease?
Thank you for this very nice summary. You mentioned the study by Pinto et al. I was astonished to learn that a B memory cell and mabs generated during a SARS-CoV infection in 2003, conferred neutralizing activity by binding to an N-type glycan of the spike protein of SARS-CoV-2. Would you think that this may form some sort of hidden herd immunity? Nearly a week ago Grifoni (Sette group) have shown in a nice Cell paper that cross-reactive CD4 and CD8 T cells forSARS-CoV2 are present in people never exposed to this virus. This people may have suffered from a corona virus cold of the upper respiratory tract by OC43 for example. Would you think that parts of our populations are protected because they developed crossreactive T cells and antibodies against the common cold coronaviridae?
Thats a really great article, thank you, changed my perspective about this pandemic …
It takes time to ensure there are no undesired side effects to humans. They are working as fast as humanly possible for this disease. Research is a science that is proven to work with time. Clinical tests are coming. There are phases of research for a reason and safety and efficacy are huge for research. If you use a drug that hasn’t been properly tested in humans and just inject people, there is NO way to know if it will actually work as intended until proper testing has been done.
A great article as usual and very informative. While mAbs are used for treatment of many serious diseases now I do not not think a mAb or mixture of mAbs would ever be a frontline treatment for Covid19 because of the cost to develop and manufacture and store them and because they cannot be reasonably manufactured and stored in large enough quantities to forestall or generally treat a pandemic. However after a vaccine is being employed worldwide they would be very useful as a tool of last resort to keep people off a ventilator and save their lives yet this would also likely add another $50K charge to their treatment bills – whether they worked or not. The most amazing thing to me about this study and the science presented is how quickly it was done and published with such molecular detail from a relatively obscure institute in China. The labs and universities in China have been making great strides toward becoming a major force in molecular and bio-sciences as exemplified in the present studies. As more and more of the papers published in Science and Nature originate from China I would guess that in the not to distant future Dr Collins will find himself directing the second best institute for biosciences/health in the world.
Very informative and helpful.
The researchers have to work as fast possible in development of covid-19 treatment drugs
thanks for sharing this wonderful article …
This was very informative article with beautiful content …
I am a 70 year old female with several pre existing conditions, Including anemia, bradycardia, asthma. I had the infusion treatment 3 days ago, the day I was diagnosed. I feel absolutely fine. Temperature normal, light cough, minimal fatigue. It worked. I don’t even feel sick.
Thank you for sharing this article..
Myself and my Husband had this treatment for Covid-19. We took it under 10days of sickness. 24 hours we felt alot better. I believe it saved my life, I was very sick. Thank you for this article.